‘Artificial wombs’ may soon see human trials: Report
A team of US researchers, who, in 2017, first carried out the ‘artificial womb’ experiment, are now seeking Food and Drug Administration (FDA) approval for its tests on humans, according to a report.
New York, Sep 20 (IANS) A team of US researchers, who, in 2017, first carried out the ‘artificial womb’ experiment, are now seeking Food and Drug Administration (FDA) approval for its tests on humans, according to a report.
The regulators are expected to consider clinical trials of the system which mimics the womb, and could reduce deaths and disability for babies born extremely premature, Nature reported.
According to the World Health Organisation, preterm birth is the largest cause of death and disability in children under five.
In 2020, there were about 13.4 million such births worldwide.
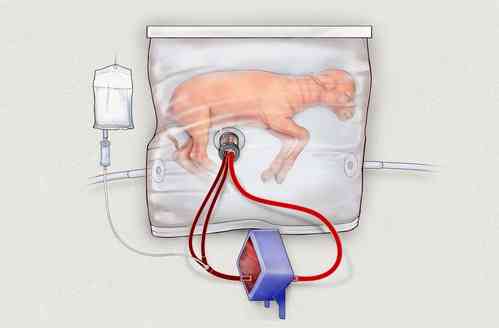
In 2017, scientists at the Children’s Hospital of Philadelphia (CHOP) in Pennsylvania kept a developing lamb alive for 28 days in a sterilised bag filled with fluid, where it received amniotic fluid, medicine and oxygen through tubes connected to umbilical cord tissue.
The experiment showed that the lamb had positive growth in its lung, GI tract and brain development.
Now, the team at CHOP have sought approval for the first human clinical trials of the device they’ve been testing, named the Extra-uterine Environment for Newborn Development, or EXTEND.
The team has emphasised that the technology is not intended -- or able -- to support development from conception to birth. Rather, it may simulate some elements of a natural womb and will increase survival, improve outcomes for extremely premature babies, the report said.
"If it’s as successful as we think it can be, ultimately, the majority of pregnancies that are predicted at-risk for extreme prematurity would be delivered early onto our system rather than being delivered prematurely onto a ventilator," Alan Flake, a foetal surgeon at CHOP who has been leading the effort, was quoted as saying in a 2017 video.
Flake is among several of the CHOP team who joined a start-up company, Vitara Biomedical in Philadelphia. It has since raised $100 million to develop EXTEND.
Meanwhile, the FDA’s meeting of independent advisers aims to discuss regulatory and ethical considerations and what human trials for the technology might look like.
"This is definitely an exciting step and it’s been a long time coming," says Kelly Werner, a bioethicist and neonatologist at Columbia University Medical Center in New York City, was quoted as saying.
"Clinicians who work with premature babies will be closely following this meeting," she said.


 IANS
IANS 










